Slip force test protocol for syringes
Recommended machine model:LS5
Origin: USA
Compliance with FDA 21 CFR Part 11 requirements
Standard:ISO 11040-4、ISO 7886-1
Typical users: Suzhou Bidi Medical, Tianjin Novo Nordisk, Hangzhou Pfizer, Shanghai Xi Pharmaceutical
Origin: USA
Compliance with FDA 21 CFR Part 11 requirements
Standard:ISO 11040-4、ISO 7886-1
Typical users: Suzhou Bidi Medical, Tianjin Novo Nordisk, Hangzhou Pfizer, Shanghai Xi Pharmaceutical
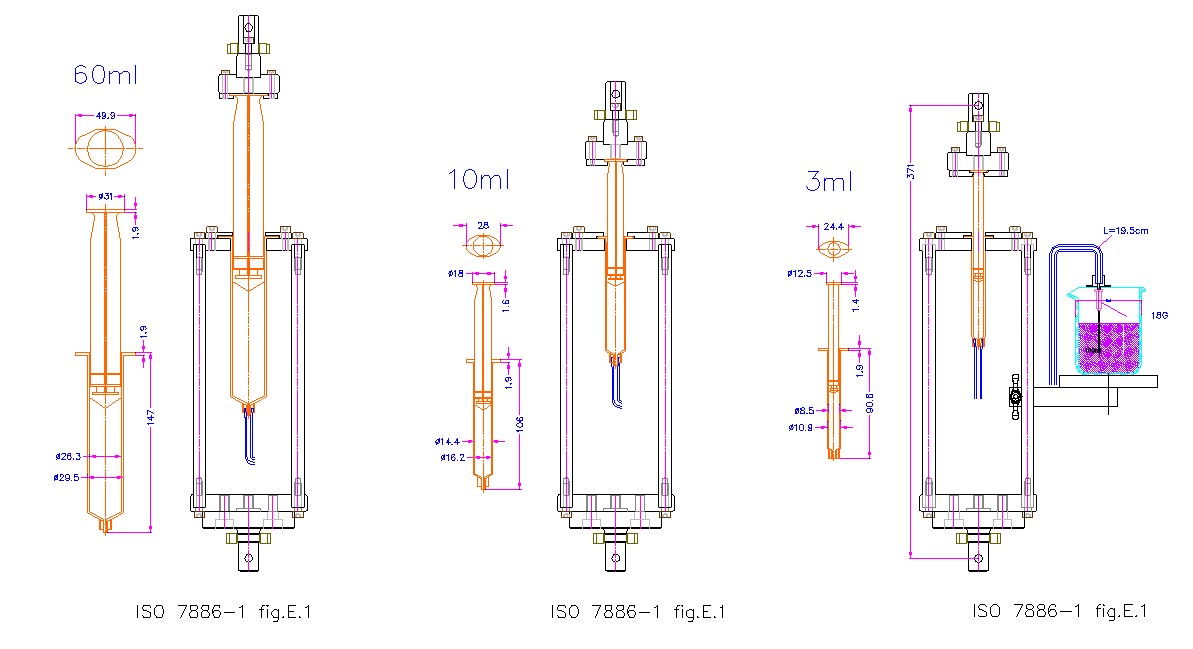
Syringe skid force test in accordance with iso 7886-1 and ISO11040-4
The test plan includes:
High precision material testing machine LS5 host
500N force sensor
A set of special fixed fixture suitable for different specifications of syringes
A flask with a scale
A versatile set of test analysis software that meets FDA CFR21 part 11 requirements

Next:
Automation industry


